Approval for biocidal product
Manufacture, import, sales, and distribution of products that do not receive the approval of the MoE are banned.
■ Conditions for Approval
1. Shall not adversely impact human/animal health or the environment
2. Shall be an active substance used within the approved scopes or deemed to have low risk as MOE notifies
3. Sufficiently effective/efficacious for removing harmful life
4. Shall not induce tolerance in harmful life
5. If used for the purpose of removing vertebrate, it shall not induce "unnecessary pain" in the vertebrate.
6. Shall use the safe containers or packaging with the standards regulated by the Environmental decree
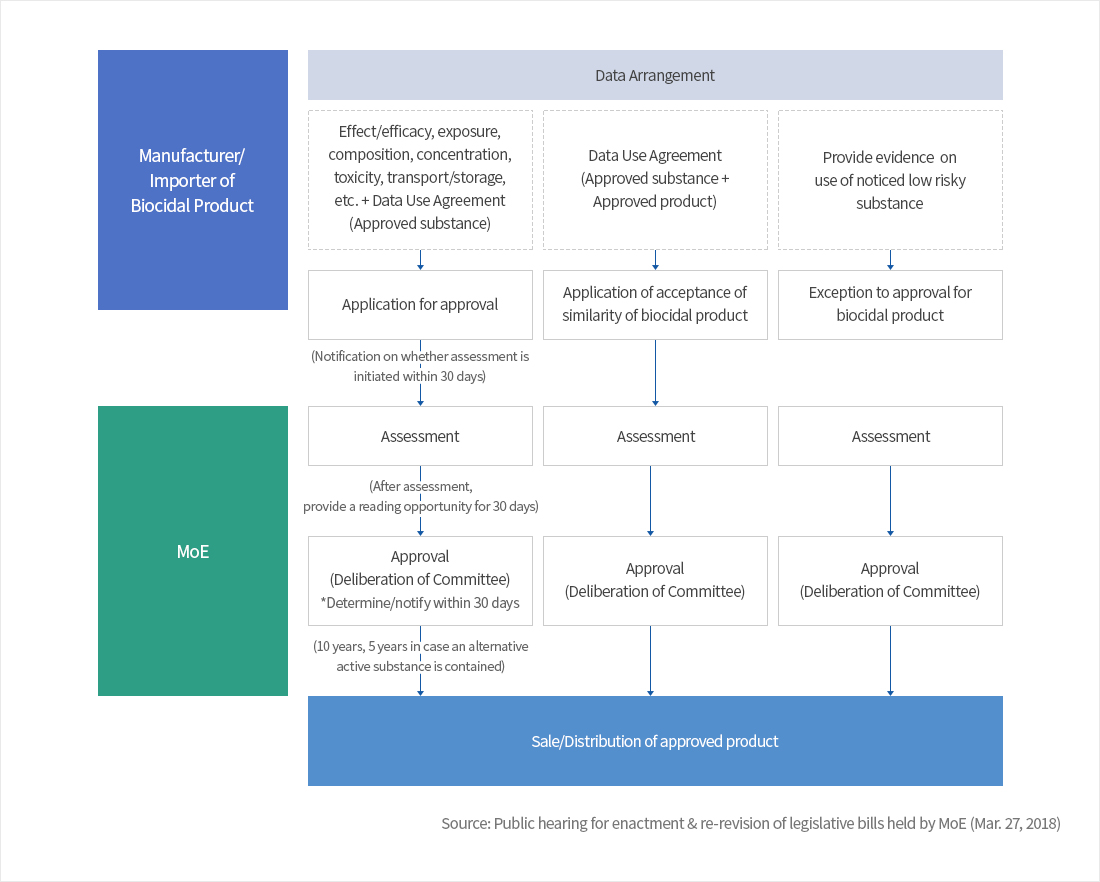
Approval procedure of biocidal product
- (Acceptance of similarity) If a biocidal product can be proved on the similarity with other approved biocidal product, the approval procedure can be way more simplified.
Labeling Requirements
1. Components and mixing ratio of all substances including active substances contained in biocidal products
2. Name and address of manufacturer or importer of biocidal products
3. Direction for dangers of uses and first aid methods
4. Direction for the safe disposal and expiry date
5. If the nanomaterials are intentionally contained in the product, the name of the substance, purpose of use and uses required